DECREASED RISK
HUMIRA for Non-Infectious Intermediate, Posterior, and Panuveitis
What is HUMIRA?
HUMIRA is a prescription medicine used to treat non-infectious intermediate (middle part of the eye), posterior (back of the eye), and panuveitis (all parts of the eye) in adults and children 2 years of age and older.
HUMIRA Results in Adults with Non-Infectious Uveitis
HUMIRA increased the time non-infectious uveitis* was controlled.
In a study of adult patients with active, non-infectious intermediate, posterior, and panuveitis, disease control was defined by 4 combined measurements—the first 3 contribute to what’s commonly known as a flare:
1
Cloudiness in the middle of the eye, known as vitreous haze.
2
Inflammatory cells in the front of the eye.
3
New lesions on the back of the eye.
4
Maintenance of visual clarity (ability to read an eye chart at a distance).
IN THE SAME CLINICAL STUDY OF ADULT PATIENTS WITH ACTIVE DISEASE

HUMIRA patients experienced 50% decreased risk of a combination of flare and loss of visual clarity.

WEEKS
Differences in disease control were seen as early as 6 weeks for patients taking HUMIRA.
HUMIRA RESULTS IN CHILDREN 2 AND OLDER WITH NON-INFECTIOUS UVEITIS*
In a clinical study of children 2 years of age and older with non-infectious uveitis* who had been previously diagnosed with juvenile idiopathic arthritis (JIA), HUMIRA in combination with another drug called methotrexate (MTX) was proven to increase the time non-infectious uveitis* was controlled.
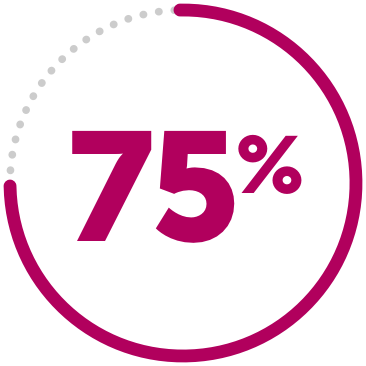
DECREASED RISK
75% decreased risk of treatment failure compared to MTX alone.

REDUCED TOPICAL STEROID USE
HUMIRA is not a steroid, and can reduce topical steroid use in children 2 and older with non-infectious uveitis.*
Individual results may vary.
*HUMIRA (adalimumab) is approved for the treatment of non-infectious intermediate, posterior, and panuveitis in adults and children 2 years of age and older.
Common side effects of HUMIRA
Common side effects of HUMIRA include injection site reactions (pain, redness, rash, swelling, itching, or bruising), upper respiratory infections (sinus infections), headaches, rash, and nausea. These are not all of the possible side effects with HUMIRA. Tell your doctor if you have any side effect that bothers you or that does not go away.
Read about the Important Safety Information for HUMIRA.
HUMIRA Dosing for Non-Infectious Uveitis in Adults
Important: You must pay attention to your starting dose – it is different than your maintenance dose and is a critical part of taking HUMIRA as prescribed.† Make sure you have been shown how to inject HUMIRA before you do it yourself. Your first injection must be given under the supervision of a health care professional. Remember to keep your HUMIRA refrigerated in its original container until ready for use.
Starting Dose
Administered on Day 1: 80 mg
Maintenance Dose
Administered every other week starting on Day 8: 40 mg
Always follow your doctor's instructions about when and how often to take HUMIRA.†
For more information, refer to the Patient Instructions for Use and the Medication Guide located inside the HUMIRA carton, and within the Full Prescribing Information.
If you experience any adverse reactions or discomfort when taking HUMIRA, discuss them with your doctor right away.
HUMIRA Dosing for Non-Infectious Uveitis in Children 2 years of age and older
Important: The doctor will take your child's weight into consideration when deciding the appropriate dose. Your child’s first injection must be given under the supervision of a health care professional. Remember to keep your HUMIRA refrigerated in its original container until ready for use.
Recommended dosing varies depending on your child’s body weight. Always follow the doctor’s instructions about when and how often your child should take HUMIRA.†
For children weighing 10 kg (22 lbs) to less than 15 kg (33 lbs), the recommended dose is 10 mg every other week.
For children weighing 15 kg (33 lbs) to less than 30 kg (66 lbs), the recommended dose is 20 mg every other week.
For children weighing 30 kg (66 lbs) and greater, the recommended dosage is 40 mg every other week.
HUMIRA is given by an injection under the skin.
HUMIRA has not been studied in patients with pediatric uveitis less than 2 years of age or in patients with a weight below 10 kg (22 lbs).
Your child's doctor will follow up with you on a regular basis. For more information, refer to the Patient Instructions for Use and the Medication Guide located inside the HUMIRA carton, and within the Full Prescribing Information.
Your child should continue taking HUMIRA as directed by your child's doctor. Remember, HUMIRA is a treatment, not a cure. Your child's doctor can tell you if and when your child should stop taking HUMIRA. If your child experiences any adverse reactions or discomfort when taking HUMIRA, discuss them with their doctor right away.
†Refer to the Patient Instructions for Use to learn about the HUMIRA Pen and prefilled syringe.

How does HUMIRA work?
The protein TNF-alpha is believed to be part of your body’s natural processes involved in creating inflammation, and in some cases, excess inflammation. When used as prescribed, HUMIRA targets and blocks TNF-alpha, which can help reduce the excess inflammation associated with non-infectious uveitis.
